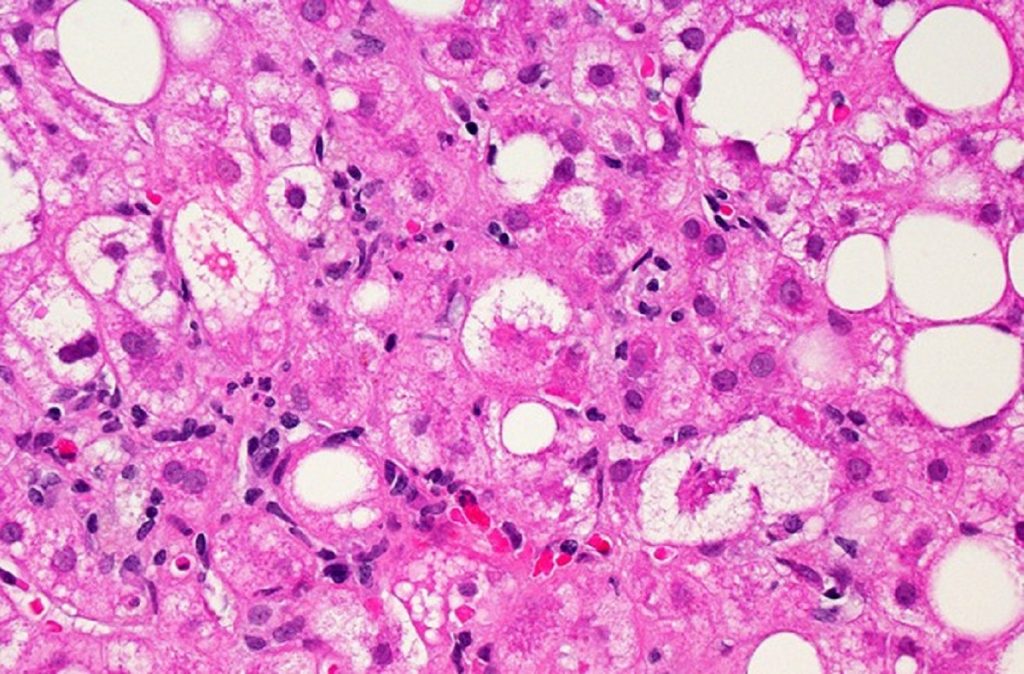
When the FDA rejected Intercept Pharmaceuticals’ drug candidate for non-alcoholic steatohepatitis (NASH) two years ago, the agency didn’t ask for another clinical trial. It said it wanted more data from the ongoing Phase 3 study. That study has accrued more safety and efficacy data, and Intercept now says these results support a second shot at seeking FDA approval. A […]