A Risky Monkeypox Vaccine Is Looking Better All the Time
The transition from Monkeypox Inoculation Plan A to Monkeypox Inoculation Plan B has been a smashing success—at least, if you ask federal officials. Just a few weeks ago, the U.S. had nowhere near enough of the Jynneos vaccine to doubly dose even a quarter of the Americans at highest risk of monkeypox, roughly 1.6 million men who have sex with men. Now that the administration has asked that every dose of Jynneos be split into five and delivered a different way, between the layers of the skin, the party line has changed. “Everyone that wants to get vaccinated within that group is going to have an opportunity to get vaccinated” by September’s end, Robert Fenton, the White House’s monkeypox czar, said on a podcast last week.
But this new strategy of intradermal dosing “is a gamble,” says Caitlin Rivers, an epidemiologist at Johns Hopkins, and its weaknesses are already beginning to show. It may be high time to start acting on a fallback plan for our fallback plan, should Plan B’s high-stakes wager not pay off.
The Plan Cs on the table aren’t very palatable—which is probably why they’re Plan Cs. One option, largely dismissed early on, could entail turning to ACAM2000, a hypereffective smallpox shot, with sometimes dangerous side effects, that the U.S. has stockpiled in spades. Already, three jurisdictions, including the state of California, have ordered more than 800 doses of ACAM from the government, according to Timothy Granholm, a spokesperson for HHS.
Simply anticipating the possibility of Plan B’s failure might count as atypical for modern American public health—getting ahead of the virus du jour, rather than taking a reactive stance, says Stella Safo, an HIV physician in New York. Too often in the past few years, the institutions of public health have observed rather than acted, allowing SARS-CoV-2, and now monkeypox, to run roughshod over the American populace. “It would be really nice to not be saying, ‘Let’s wait and see,’” Safo told me. ACAM2000 may not be the country’s best or safest option for curtailing monkeypox, but the risk of not considering it may soon outweigh the risks of the shot itself.
There’s a world in which the U.S. didn’t even need a Monkeypox Inoculation Plan B. Had U.S. leaders been willing to invest resources in heading off the pathogen, by offering aid to countries where the virus has been endemic for decades or by focusing earlier this year on tests, treatments, vaccines, and public communications, maybe America’s original immunization plan—using the full, subcutaneous Jynneos dose—would have been all the nation needed on the injection front.
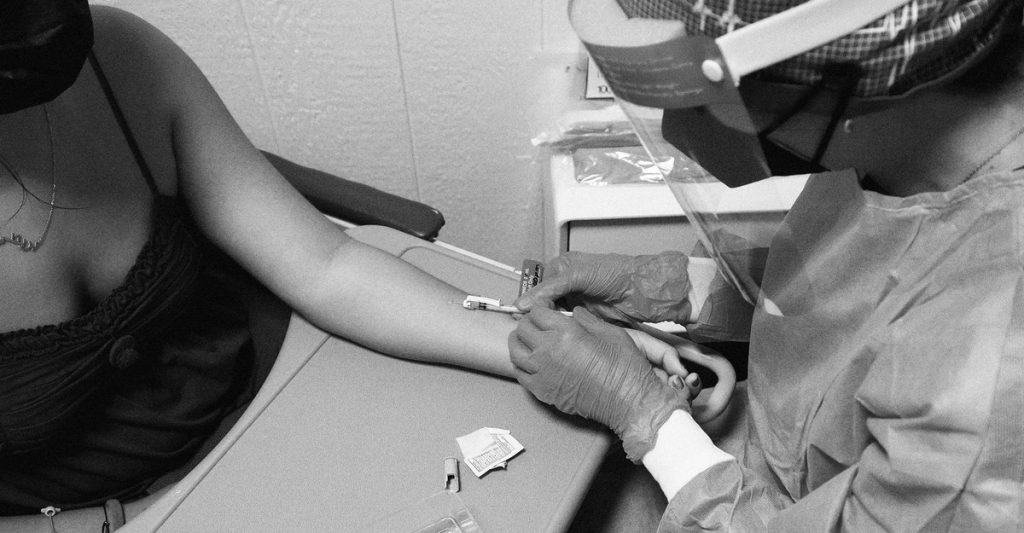
That didn’t happen, and instead the country adopted intradermal delivery, without real clarity on how well such doses might guard against infection, transmission, or disease. The notion that intradermal shots will work as hoped rests on a “chain of assumptions,” says John Beigel, an immunologist at the National Institute of Allergy and Infectious Diseases, several of which may not hold during a large, fast-spreading outbreak that’s tightly linked to sex—a poorly studied form of monkeypox transmission. Jynneos’s original approval was based on an antibody analog of protection, rather than efficacy against bona fide illness. And the FDA’s authorization of intradermal shots rests on a single study, which didn’t directly check the vaccine’s ability to stave off disease, either. The study also enrolled only healthy adults, most of them white—a poor reflection of the population now being hit. It’s a “big leap” to build a nationwide vaccine campaign on just those results, says Sri Edupuganti, a vaccinologist at Emory University and one of the study’s authors. (Beigel is now designing a clinical trial that will reevaluate the intradermal route among participants more relevant to the current outbreak. He and his team will also test one-tenth intradermal doses, which could further stretch supply.)
The intradermal plan has logistical challenges, too. Administering in-skin shots requires extra training and special needles, burdening already stressed staff, especially in low-resource regions. Several jurisdictions are struggling to extract more than three or four doses from some vials, rather than the government’s promised five—a shortchanging of those hoping to increase their stocks by a clean 400 percent. Plus, some bottle caps are breaking before all the doses are withdrawn. Intradermal vaccination can also come with grating side effects, including redness and swelling that can stick around for days, potentially deterring people from returning for the essential second shot.
Fenton, from the White House, noted in a press briefing last week that the switch to intradermal “increased our supplies significantly without compromising safety or effectiveness.” But that assertion seems “disingenuous at best,” says Gregg Gonsalves, an epidemiologist and AIDS activist at Yale’s School of Public Health. Even the CEO of Bavarian Nordic, the vaccine’s manufacturer, criticized the FDA’s pivot as too hasty. (The FDA attempted to counter the company’s criticisms.)
Meanwhile, demand may continue to grow, especially if the epidemic starts to concentrate less among men who have sex with men. “The longer the outbreak lasts, the longer you have for jumping to other populations,” Gonsalves told me. College campuses, reopening now, “seem like the most obvious next stop.” And “if this gets into other networks,” says Ina Park, a sexual-health expert at UC San Francisco, Plan B “just won’t be enough.”
Equity, too, is becoming an issue. “If we lived in a world where we had plenty of vaccine, you would go with subcutaneous,” Beigel told me. But in North Carolina, for instance, where 70 percent of monkeypox cases have been among Black men, some two-thirds of the subcutaneous shots administered before August 8 went to people who are white; similar skews have been noted in New York City. Now “Black and brown gay men are really angry,” says Kenyon Farrow, a writer and public-health activist based in Ohio. “They watched white gay men get full doses … and now they feel like they are getting less of a dose.” Farrow has pushed for everyone to get at least one subcutaneous shot—a strategy that advocates in New York City also back—but the Biden administration seems set on moving all jurisdictions onto the intradermal route.
Mapping out yet another vaccination strategy won’t address all of these problems. (And no matter what, the administration should keep ordering more Jynneos, stat.) But the forecast for fall is murky. And should the present situation worsen, a fresh tactic could give the U.S. a head start—something the country hasn’t had on the public-health playing field in a while.
Already, some experts are mulling the nuclear option: ACAM2000, the smallpox shot that the government has been hoarding to counter a potential bioterrorism attack. Doses of the vaccine are available by the many millions, and thought to be both effective and durable. It’s also, Edupuganti told me, “one of the vaccines with the highest amount of adverse reactions,” occasionally triggering side effects as serious as heart inflammation. The shot contains a replicating virus, and shouldn’t be taken by immunocompromised people, including many of those who are living with HIV. And just about everyone who gets the shot sprouts an oozy lesion at the injection site that can pass the vaccine virus to others. Against something like smallpox—a far more contagious virus that killed up to 30 percent of its victims—ACAM2000 would be “a no-brainer,” says Rafi Ahmed, a vaccinologist at Emory University. With monkeypox, though, Johns Hopkins’s Rivers told me, the risk-benefit calculation “is really hazy.”
It’s not time to trot out ACAM yet, Safo, the New York physician, told me. But maybe autumn will bring many more cases. Maybe monkeypox’s symptoms could grow more severe. Maybe the virus will start to surge in new populations. Maybe intradermal Jynneos will fall short in effectiveness or safety. In any case, containment with the current tools isn’t a guarantee. “If things do get out of control,” Ahmed told me, “you want to have some ACAM stocks ready to go.” No clear, perfect threshold can yet denote “out of control.” Still, a trend toward a worse outbreak would inch the country closer to tapping into its ACAM2000 supply, Park told me: “I don’t think we have another choice.” Which means that the FDA and CDC should probably start poring over the ACAM data now, Rivers said.
Resorting to ACAM2000 will also put the onus on officials to explain to the public what they’re getting into. If some are balking at intradermal shots, people further back in line could reasonably wonder why they’ve been stuck with a less-safe vaccine, Farrow pointed out. There could be a middle ground worth testing in a clinical trial: one shot of Jynneos, via either administration route, followed by a dose of ACAM2000, says Stephen Goldstein, a virologist at the University of Utah. One 2019 study hints that this shot, chaser approach could shrink infectious lesions, as well as cut down on ACAM2000’s side effects, while still offering an immunological boost—though that trial used two subcutaneous Jynneos doses first. In any case, the government would do well to pursue more options, even enroll people in trials comparing the different vaccines, Gonsalves told me. And transparency is tantamount. “Back in the days of AIDS,” he said, “many of us were saying, as new drugs were coming online, we wanted access and answers” about the options at hand. Right now, the nation’s short on both.
That “we’re even having to ask these questions about ACAM,” Farrow told me, is a sobering reminder that “we didn’t get our shit together” early on. Instead, the U.S. has backed itself into having to reckon with its appetite for risk. Being too cautious with vaccines could allow the outbreak to further balloon; being too reckless with shots could compromise public trust. The administration firmly contends that Jynneos remains “the best available option,” according to Granholm, the HHS spokesman. (That said, ACAM2000 “is available upon request,” he told me.)
Such a position may feel like the safe one—it potentially sidesteps the gnarliness of ACAM. But perhaps it’s actually dicier, because it’s not properly preparative. “We can’t just say intradermal is going to solve all of our problems,” Park told me. Although the hope is that the country’s ACAM supply can stay stashed away, we need to be ready to use it, and quickly, should the need arise. If the country once again waits until “we’re in a pinch” to act, Rivers told me, “it’s going to be too late.”