Minnetronix commercializes its own product: a minimally invasive deep brain access port – MedCity News
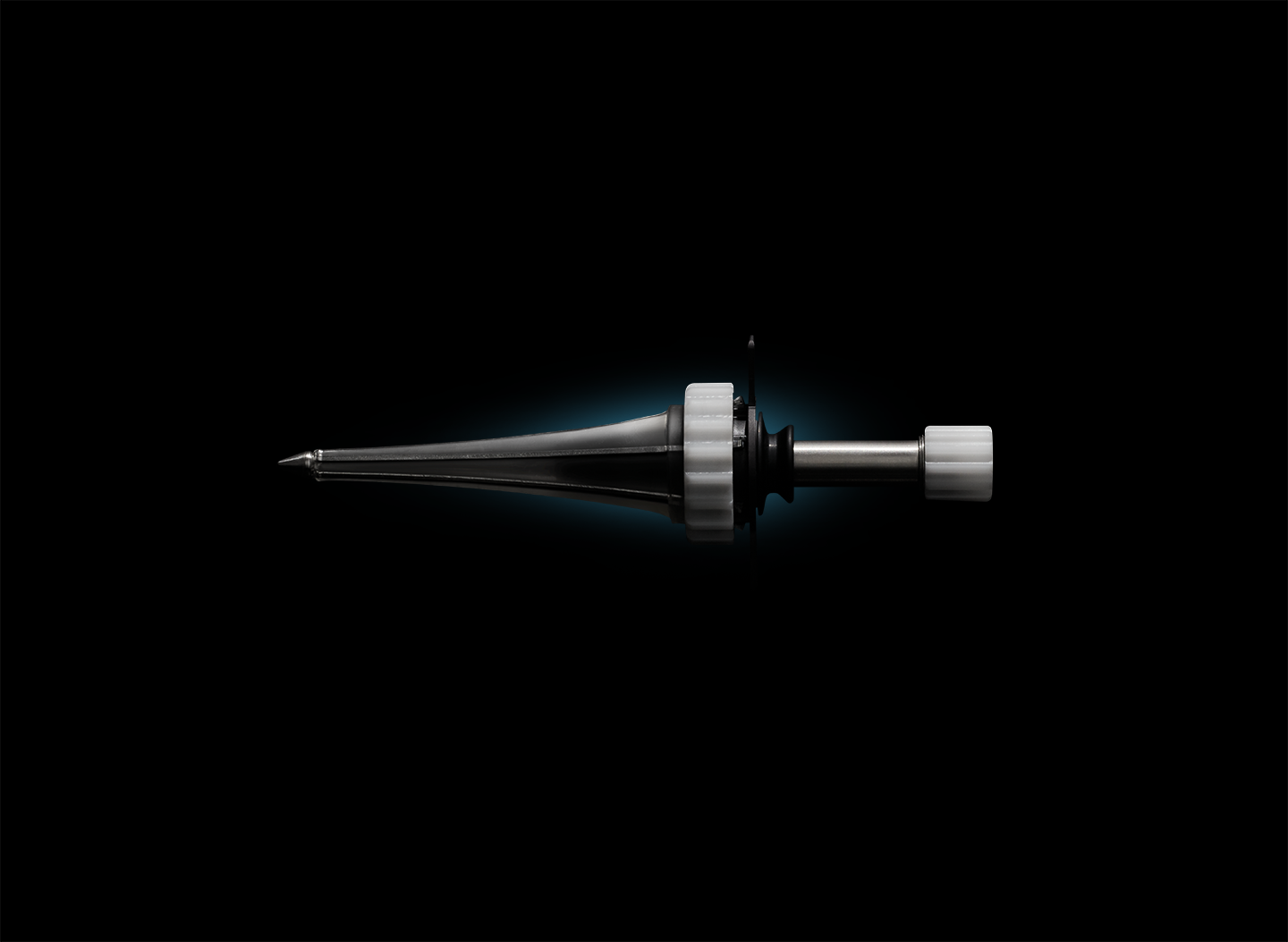
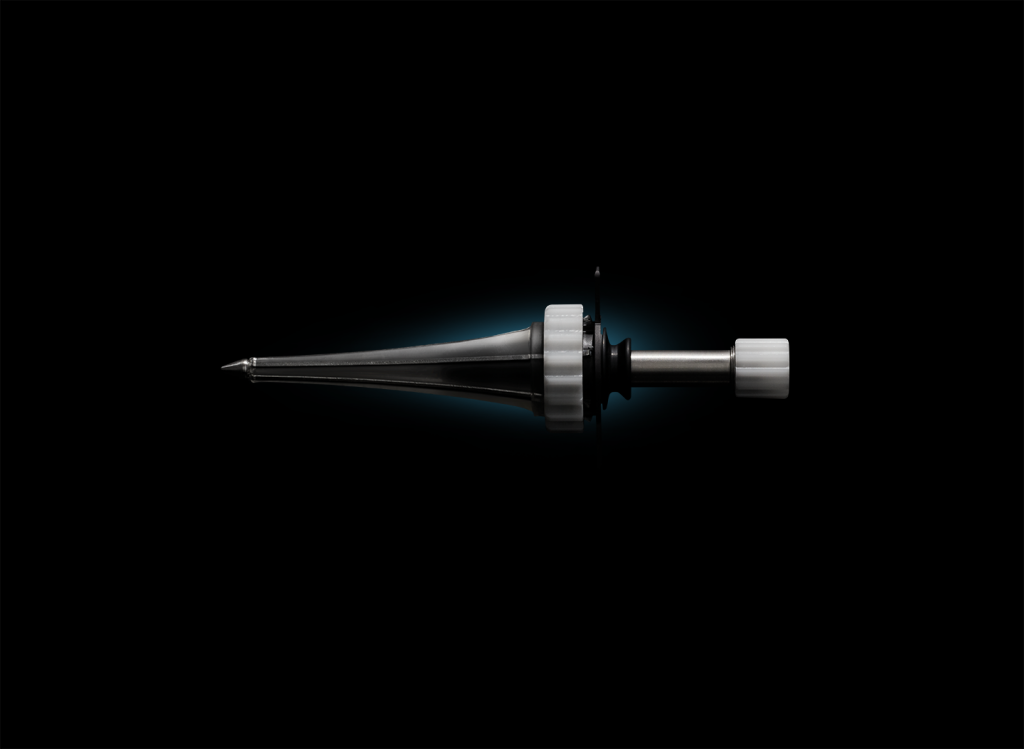
Contract developer Minnetronix Medical recently announced that it has developed and commercialized its own product, an expandable deep brain access port called MindsEye.
The St. Paul-based company has been around since 1996. During those 26 years, Minnetronix has manufactured devices across four key technology segments: optical systems, radio frequency energy, stimulation and active wearables, and fluid and gas management. The company has expertise developing products across most areas within medicine — from neurology to cardiology to gastroenterology to intensive care — Matt Adams, general manager at Minnetronix, said in an interview.
Until now, Minnetronix was known for developing products for other companies. Some of these include Medtronic, Abbott, United Therapeutics, Boston Scientific and Minerva Surgical.
In the past, these and other companies would come to Minnestronix with product ideas, and it would conduct research and development on their behalf. Now, companies can still do this, but they can also come to Minnetronix to get a product that is completely done — conceived, designed, developed, manufactured and taken through regulatory clearance.
“In the case of MindsEye, if you were a company that had a salesforce that’s focused in the neuro space, we have a product that is completely finished,” Adams said. “We can drop straight into your sales bag and will continue to work together to manufacture and improve it as time goes on.”
That is what is happening with Minnetronix’s partner for MindsEye — Mizuho America — which will distribute the device throughout the U.S.
Certain forms of stroke and cancer cause lesions that are deep in the brain, and removing them can be a very invasive and dangerous process. Adams declared that MindsEye is the only expandable, minimally invasive deep brain access port on the market. Thereby, it addresses an unmet need in the neurology market.
The port provides deep brain access and visualization during surgeries to treat stroke and cancer. It removes lesions with “a lot less damage” to the brain than the more invasive ports that came before from companies like Medtronic and Boston Scientific, Adams claimed.
MindsEye earned its clearance from the Food and Drug Administration in 2020, and is reimbursable. Its first live human use cases were conducted at Tulane Medical Center by neurosurgeons Dr. Art Wang and Dr. Johnny Delashaw. Tulane now regularly uses the device within its neurosurgery department.
Having developed MindsEye, Minnetronix can take its future partnerships “to a whole new level,” because device companies now have an option to get a finished product, Adams declared. The company plans to continue conceptualizing and developing more products in-house in the future.
The next in-house product in Minnetronix’s portfolio is Neurapheresis, which Adams described as “a very novel system that is essentially like dialysis for your cerebral spinal fluid.” The company recently completed two prospective, multicenter U.S. clinical trials for the product, and Adams said Minnetronix is currently looking for a partner to distribute the device.
Photo source: Minnetronix Medical