How Will the Prescription Drug Provisions in the Inflation Reduction Act Affect Medicare Beneficiaries?
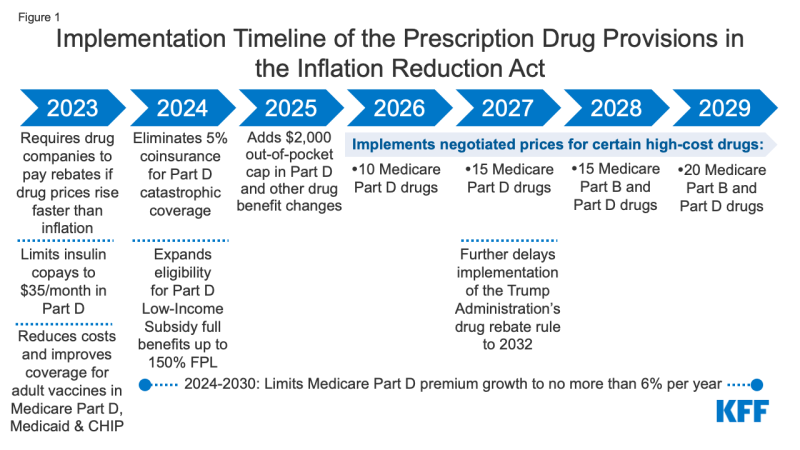
On August 16, 2022, President Biden signed into law the Inflation Reduction Act of 2022, which includes a broad package of health, tax, and climate change provisions. The law includes several provisions to lower prescription drug costs for people with Medicare and reduce drug spending by the federal government. These provisions will take effect beginning in 2023 (Figure 1). This brief examines the potential impact of these provisions for Medicare beneficiaries nationally and by state.

Figure 1: Implementation Timeline of the Prescription Drug Provisions in the Inflation Reduction Act
The Inflation Reduction Act includes two policies that are designed to have a direct impact on drug prices:
- Requires the federal government to negotiate prices for some high-cost drugs covered under Medicare. Medicare Part D and Part B drug spending is highly concentrated among a relatively small share of covered drugs, mainly those without generic or biosimilar competitors. Under the Inflation Reduction Act, brand-name and biologic drugs without generic or biosimilar equivalents covered under Medicare Part D (retail prescription drugs) or Part B (administered by physicians) that are among the highest-spending Medicare-covered drugs and are nine or more years (small-molecule drugs) or 13 or more years (biologicals) from FDA approval are eligible for negotiation. The number of negotiated drugs is limited to 10 Part D drugs in 2026, another 15 Part D drugs in 2027, another 15 Part B and Part D drugs in 2028, and another 20 Part B and Part D drugs in 2029 and later years.
- The number of Medicare beneficiaries who will see lower out-of-pocket drug costs in any given year under this provision, and the magnitude of savings, will depend on which drugs are subject to negotiation, the number of Medicare beneficiaries who use those drugs, and the price reductions achieved through the negotiation process relative to prices that would have been applied in the absence of the new law.
- Requires drug manufacturers to pay rebates to Medicare if they increase prices faster than inflation for drugs used by Medicare beneficiaries. From 2019 to 2020, half of all drugs covered by Medicare had price increases above the rate of inflation over that period (which was 1%, prior to the recent surge in the annual inflation rate), and among those drugs with price increases above the rate of inflation, one-third had price increases of 7.5% or more, the annual inflation rate in early 2022. The inflation rebate provision will be implemented in 2023, using 2021 as the base year for determining price changes relative to inflation. (The legislation originally included drug use by people with private insurance in the calculation of the rebate, but that language was dropped based on a ruling by the Senate parliamentarian that it did not comply with budget reconciliation rules.)
- The number of Medicare beneficiaries who will see lower out-of-pocket drug costs in any given year under this provision will depend on how many beneficiaries use drugs whose prices increase more slowly than would otherwise occur and the magnitude of price reductions relative to baseline prices. This provision could have spillover effects on people with private insurance if it results in slower price growth for drugs covered by private insurance.
The Inflation Reduction Act includes several provisions that will reduce out-of-pocket spending for Medicare beneficiaries:
- Caps Medicare beneficiaries’ out-of-pocket spending under the Medicare Part D benefit, first by eliminating coinsurance above the catastrophic threshold in 2024 and then by adding a $2,000 cap on spending in 2025. The law also limits annual increases in Part D premiums for 2024 to 2030 and makes other changes to the Part D benefit design. Under current law, the catastrophic threshold is based on the amount beneficiaries themselves pay out-of-pocket plus the value of the manufacturer discount on the price of brand-name drugs in the coverage gap phase. In 2022, the catastrophic threshold is set at $7,050, and beneficiaries pay about $3,000 out of pocket for brand-name drugs before reaching the catastrophic coverage phase, where they pay 5% coinsurance on their drugs until the end of the year. Based on current estimates, beneficiary out-of-pocket spending at the catastrophic coverage threshold is estimated to increase from $3,000 in 2022 to roughly $3,100 in 2023 and $3,250 in 2024.
- In 2020, 1.4 million Medicare Part D enrollees without low-income subsidies had annual out-of-pocket drug spending of $2,000 or more, including 1.3 million enrollees who had spending above the catastrophic coverage threshold (which equaled roughly $2,700 in out-of-pocket costs that year for brand-name drugs alone). (See Table 1 for state-level estimates.) Among these 1.4 million enrollees, most (1.0 million or 69%) spent between $2,000 and $3,000 out of pocket, while roughly 0.3 million (19%) had spending of $3,000 up to $5,000, and 0.2 million (11%) spent $5,000 or more out of pocket.
- These estimates of how many beneficiaries will be helped by capping out-of-pocket drug spending under Medicare Part D starting in 2024 are conservative because they do not account for expected increases in annual out-of-pocket drug spending between 2020 and 2024/2025, the increase in the number of beneficiaries on Medicare, or higher utilization and spending associated with the increased affordability of prescription drugs due to this benefit improvement.
- Capping out-of-pocket drug spending under Medicare Part D will be especially helpful for beneficiaries who take high-priced drugs for conditions such as cancer or multiple sclerosis. For example, in 2020, among Part D enrollees without low-income subsidies, average annual out-of-pocket spending for the cancer drug Revlimid was $6,200 (used by 33,000 beneficiaries); $5,700 for the cancer drug Imbruvica (used by 21,000 beneficiaries); and $4,100 for the MS drug Avonex (used by 2,000 beneficiaries).
- Limits cost sharing for insulin to $35 per month for people with Medicare, beginning in 2023, including covered insulin products in Medicare Part D plans and for insulin furnished through durable medical equipment under Medicare Part B. (A provision to limit monthly insulin copays for people with private insurance did not receive the 60 votes needed to remain in the bill after being ruled out of compliance with reconciliation rules by the parliamentarian and was removed from the legislation prior to passage.)
- 3.3 million Medicare Part D enrollees used an insulin product in 2020 (the most recent data available), including 1.7 million enrollees without low-income subsidies who spent $54 on average per insulin prescription that year. The number of Medicare beneficiaries who will pay less out of pocket for insulin beginning in 2023 will depend in part on whether they are currently enrolled in a Part D plan that is participating in an Innovation Center model in which participating plans cover selected insulin products at a monthly copayment of $35.
- Eliminates cost sharing for adult vaccines covered under Medicare Part D, as of 2023, and improves access to adult vaccines under Medicaid and CHIP.
- 4.1 million Medicare beneficiaries received a vaccine covered under Part D in 2020, including 3.6 million who received the vaccine to prevent shingles. (See Table 1 for state-level estimates.)
- The Medicaid and CHIP provision improves vaccine coverage for Medicaid-enrolled adults because vaccine coverage is optional and varies by state. According to a recent survey, half of states (25) did not cover all vaccines recommended by the Advisory Committee on Immunization Practices (ACIP) in 2018–2019, and 15 of 44 states responding to the survey imposed cost sharing requirements on adult vaccines.
- Expands eligibility for full Part D Low-Income Subsidies (LIS) in 2024 to low-income beneficiaries with incomes up to 150% of poverty and modest assets and repeals the partial LIS benefit currently in place for individuals with incomes between 135% and 150% of poverty. Beneficiaries receiving partial LIS benefits typically pay some portion of the Part D premium and standard deductible, 15% coinsurance, and modest copayments for drugs above the catastrophic threshold, while those receiving full LIS benefits pay no Part D premium or deductible and only modest copayments for prescription drugs until they reach the catastrophic threshold, when they face no cost sharing.
- 0.4 million Medicare beneficiaries received partial LIS benefits in 2020. Annual out-of-pocket costs for these beneficiaries could fall by close to $300, on average, under the new law, based on the difference between average out-of-pocket drug costs for LIS enrollees receiving full benefits versus partial benefits in 2020. (See Table 1 for state-level estimates.)
- This provision will benefit low-income Black and Hispanic Medicare beneficiaries in particular, who are more likely than white beneficiaries to have incomes between 135% and 150% of poverty.
The Inflation Reduction Act also includes a provision to further delay implementation of the Trump Administration’s drug rebate rule until 2032, rather than take effect in 2027. The rebate rule would eliminate the anti-kickback safe harbor protections for prescription drug rebates negotiated between drug manufacturers and pharmacy benefit managers (PBMs) or health plan sponsors in Medicare Part D. This rule was estimated to increase Medicare spending and premiums paid by beneficiaries.
Discussion
High and rising drug prices are a top health care affordability concern among the general public, with large majorities of Democrats and Republicans favoring policy actions to lower drug costs. Provisions in the Inflation Reduction Act are expected to lower out-of-pocket spending by people with Medicare and lower drug spending by the federal government. Prior to consideration by the Senate, CBO estimated the prescription drug provisions would reduce the federal deficit by $288 billion over 10 years (2022-2031). CBO has not yet released a final estimate of budget effects that reflect changes made to the legislation before final passage, such as the $35 per month limit on cost sharing for insulin for people with Medicare and the removal of the provision that applied the inflation rebate to prescription drug use by people with private insurance.
The prohibition against the federal government negotiating drug prices was a contentious provision of the Medicare Modernization Act of 2003, the law that established the Medicare Part D program, and lifting this prohibition has been a longstanding goal for many Democratic policymakers. The pharmaceutical industry has argued that allowing the government to negotiate drug prices would stifle innovation. CBO has estimated that 15 out of 1,300 drugs, or 1%, would not come to market over the next 30 years as a result of the drug provisions in the reconciliation legislation.
The requirement for drug companies to pay rebates for price increases faster than inflation will help to limit annual increases in drug prices for people with Medicare and possibly also those with private insurance. While it is possible that drug manufacturers may respond to the inflation rebates by increasing launch prices, overall, this provision is expected to limit out-of-pocket drug spending growth and put downward pressure on premiums by discouraging drug companies from increasing prices faster than inflation.
Capping Medicare beneficiaries’ out-of-pocket spending under the Medicare Part D benefit – first by eliminating coinsurance above the catastrophic threshold in 2024 and then by adding a $2,000 cap on spending in 2025 – will be the first major change to the Medicare Part D benefit since 2010, when lawmakers included a provision in the Affordable Care Act to close the so-called Part D “donut hole.” A cap on out-of-pocket drug spending for Medicare Part D enrollees will provide substantial financial protection to people on Medicare with high out-of-pocket drug costs. This includes Medicare beneficiaries who take just one very high-priced specialty drug for medical conditions such as cancer, hepatitis C, or multiple sclerosis and beneficiaries who take a handful of relatively costly brand or specialty drugs to manage their medical conditions.
This work was supported in part by Arnold Ventures. KFF maintains full editorial control over all of its policy analysis, polling, and journalism activities.
Juliette Cubanski, Tricia Neuman, and Meredith Freed are with KFF. Anthony Damico is an independent consultant.